Self-organized growth of flower-like SnS2 and forest-like ZnS nanoarrays on nickel foam for synergistic superiority in electrochemical ammonia synthesis†
Abstract
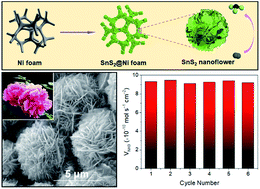
The exploration of non-noble metal catalysts toward the electrochemical nitrogen reduction reaction (NRR) is crucial for industrial-scale ammonia synthesis. Although metal sulfides have long been predicted to be electrocatalytically more efficient than other compounds, there has been no substantial progress made on them due to the difficulties in the controllable synthesis of elaborate nanostructures with optimized NRR performance. Besides, their inferior electrical conductivity is not favorable for electrocatalysis. Herein, we propose an interesting conceptual design to integrate novel metal sulfide catalysts with a fascinating conductive matrix. Through self-organized growth under solvothermal conditions, flower-like SnS2 and forest-like ZnS nanoarrays are directly formed on Ni foam with intimate adhesion. Both SnS2 and ZnS exhibit remarkable abilities in nitrogen activation, which are further enhanced by forming well-aligned nanoarrays on 3D porous Ni foam, offering a large surface area and enabling easy electrolyte permeation. Moreover, Ni foam significantly outperforms carbonaceous materials as a conductive matrix because of its far better electrical conductivity and mechanical robustness. The resulting SnS2@Ni and ZnS@Ni foams show synergistic superiority as advanced hybrid catalysts, delivering high ammonia yields and faradaic efficiencies comparable to or even better than those of noble-metal-based catalysts.
- This article is part of the themed collection: 2019 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...