Boosting the oxygen evolution reaction activity of a perovskite through introducing multi-element synergy and building an ordered structure†
Abstract
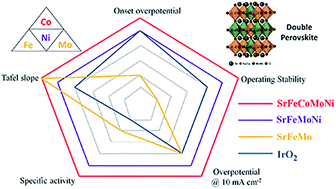
If different active sites in a catalyst have optimal binding to different reaction intermediates and short reaction paths among them, they may work cooperatively to enhance the oxygen evolution reaction (OER) activity. Based on this design principle, in this study, we start with a B-site ordered double perovskite Sr2FeMoO6−δ with poor OER activity as the host material to fulfill the requirement of a short pathway, and then, replace Mo with Ni and Fe with Co to optimize the synergistic interplay of the multi-active sites. Replacing Mo with Ni indeed dramatically enhances the OER activity and structural/operating stability. Further improvement in OER performance is realized by partial substitution of Fe with Co, leading to the development of a material with the nominal composition of Sr2Fe0.8Co0.2Mo0.65Ni0.35O6−δ, which outperforms the noble metal oxide IrO2 and is better than most of the electrocatalysts developed based on a single descriptor, such as Ba0.5Sr0.5Co0.8Fe0.2O3−δ (eg occupancy close to unity), PrBaCo2O5+δ (O 2p-band center relative to the Fermi level), and La0.5Sr0.5CoO3−δ (charge-transfer energy) in many aspects. As a universal method, combined structural and compositional tuning to create a cooperative effect among different active sites for intermediate adsorption and reaction in an ordered structure may provide a new way for the design of superior electrocatalysts for various applications.


 Please wait while we load your content...
Please wait while we load your content...