In situ structural evolution of a nickel boride catalyst: synergistic geometric and electronic optimization for the oxygen evolution reaction†
Abstract
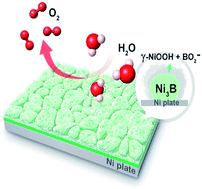
The oxygen evolution reaction (OER), as a kinetically sluggish half-reaction, plays a crucial role in the overall efficiency of some important electrochemical energy conversion systems. Herein we report the synthesis of nickel boride layers supported on a nickel plate via a direct solid boronization of the nickel substrate with amorphous boron powder. The resulting boronized nickel plate was studied systematically to illustrate its surface structural evolution and activity variation during the OER. The initial electrochemical OER testing process resulted in the in situ generation on the nickel boride of thin nanosheet films that consist of metaborate-containing oxyhydroxides, as the efficient catalytically active phase. Correspondingly, this electrochemically activated, boronized nickel plate exhibits a nearly ten-fold increase in catalytic activity compared to the nickel plate, and shows remarkable catalytic stability for over 1500 hours. The enhanced catalytic performance during electrochemical activation is attributed to synergistic catalytic effects from the thin nanosheet structural features of the oxyhydroxides (i.e., geometric optimization) and modification of the electronic structure of the oxyhydroxides by metaborates (i.e., electronic optimization). Our results provide new insights into boride-based oxygen evolution electrocatalysts, and provide a new strategy for the design of high-performance electrocatalytic materials for the OER.
- This article is part of the themed collection: 2019 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...