Ammonia synthesis from nitrogen and water at intermediate temperatures and elevated pressures by using an electrochemical hydrogen-membrane reactor with supported Ru catalysts and phosphate electrolytes†
Abstract
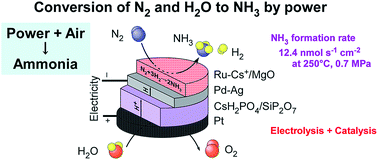
Electrochemical synthesis of NH3 from N2 and H2O with electrical power is a promising technology to convert redundant electricity to a chemical fuel. NH3 synthesis from N2 and H2O using a combination of Cs-promoted Ru catalysts, a Pd-based hydrogen-membrane cathode, a CsH2PO4/SiP2O7 electrolyte, and a Pt anode was investigated in the temperature range of 200–250 °C and pressure range of 0.1–0.7 MPa. A maximum NH3 formation rate of 12.4 nmol s−1 cm−2 (759 μg h−1 cm−2) was obtained at 250 °C and 0.7 MPa using 5 wt%-Ru/Cs+/MgO (Cs/Ru = 1 mol) for a current density of 30 mA cm−2. The corresponding current efficiency for NH3 formation was estimated to be 12%, and the remaining part of the current was confirmed to be used for H2 production. The NH3 formation rate increased upon increasing the total pressure, following thermodynamic equilibrium. 5 wt%-Ru/Cs+/CeO2 (Cs/Ru = 1 atom) was found to yield the highest formation rate of NH3 at 200–220 °C and 0.1 MPa, while Ru/Cs+/MgO yielded a higher NH3 formation rate than Ru/Cs+/CeO2 under elevated pressure conditions. Because the dissociation of linearly adsorbed molecular N2 on Ru is known to be the rate-determining step in NH3 synthesis, infrared spectroscopy was utilized to examine the linearly adsorbed N2 on the Ru sites at the adsorption equilibrium at 30 °C.


 Please wait while we load your content...
Please wait while we load your content...