“Pushing and pulling” the equilibrium through bubble mediated reactive separation for ethyl acetate production†
Abstract
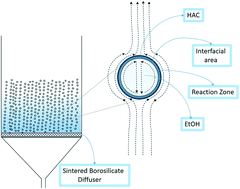
Esterification, a reaction extensively used in chemical processing, is limited by the establishment of kinetic equilibrium, i.e. marginally exer/endergonic. The reaction is generally slow with low yield making downstream separation cost intensive. A new heterogeneous contacting method for the synthesis of ethyl acetate through fine bubbles tests the hypothesis that reactive distillation can “pull” the reaction nearer to completion, reducing the downstream separation requirements. It achieves a high yield of ethyl acetate, 79.9% in 35 min, as compared with 64% conversion in 350 min using a conventional method. The kinetics of esterification reaction under bubbly flow conditions are studied – entirely different from the conventional bulk model. The alcohol is fed as vapor within the bubbles which means alcohol is always in deficit, providing an opportunity to convert an equilibrium limited reaction to nearly irreversible one. As the bubbles flow upwards, the reaction proceeds at the “skin” of the bubble. If the esterification reaction occurs at or near the microbubble interface, ethanol is in large excess in the bubble phase, as acetic acid is well below its boiling point in the liquid phase. By Le Chatelier's principle, the local excess ethanol will push the equilibrium towards completion. Similarly, removal of water and ethyl acetate via the “dry” bubble pulls the equilibrium towards completion.
- This article is part of the themed collection: 2019 Reaction Engineering in China


 Please wait while we load your content...
Please wait while we load your content...