Ni-Catalyzed electrophile driven regioselective arylative cyclization of ortho-functional diaryl acetylenes for the synthesis of pyridine and indene derivatives†
Abstract
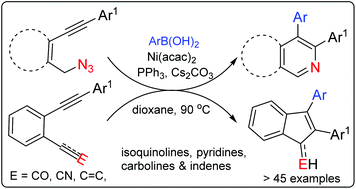
A regioselective carbonickelation followed by cyclization, an arylative cyclization, of ortho functional diaryl acetylenes is achieved apparently through an electrophile driven alkyne polarization. A series of selectively substituted di aryl isoquinoline, pyridine and indene derivatives are thus accessed from diarylacetylenes with azide, carbonyl and cyanide tethers.


 Please wait while we load your content...
Please wait while we load your content...