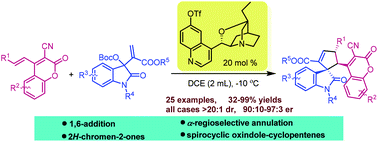
Asymmetric domino 1,6-addition/annulation reaction of 3-cyano-4-alkenyl-2H-chromen-2-ones with isatin-derived MBH carbonates: enantioselective synthesis of 3,3′-cyclopentenylspirooxindoles bearing 2H-chromen-2-ones†
Abstract
An asymmetric domino 1,6-addition/annulation reaction of 3-cyano-4-alkenyl-2H-chromen-2-ones with isatin-derived MBH carbonates was achieved. With this developed protocol, a series of 3,3′-cyclopentenylspirooxindoles bearing 2H-chromen-2-ones were obtained in moderate to excellent yields with good to excellent diastereo- and enantioselectivities (>20 : 1 dr, up to 97 : 3 er).
- This article is part of the themed collection: 2019 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...