Palladium-catalyzed dearomative arylphosphorylation of indoles†
Abstract
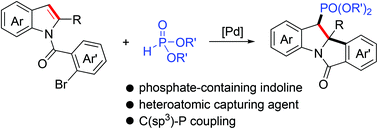
A palladium-catalyzed dearomative arylphosphorylation reaction of indoles involving C–C/C–P bond formation is developed. Through the intramolecular dearomative carbopalladation of indoles followed by an intermolecular benzyl–Pd termination with dialkylphosphite, a range of unique indolines bearing vicinal tetrasubstituted and phosphate-containing trisubstituted stereocenters are obtained in good yields. Synthetic transformations of the product show practical utilities of the reaction.
- This article is part of the themed collection: 2019 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...