Visible-light-mediated hydrodehalogenation and Br/D exchange of inactivated aryl and alkyl halides with a palladium complex†
Abstract
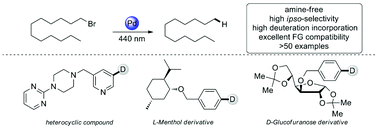
Herein, a novel photo-induced amine-free radical reductive dehalogenation of inactivated aryl/alkyl bromides and chlorides with a palladium complex is described, which reveals excellent functional group compatibility and broad substrate scope. Extensional transformations for reductive cyclization, dehalogenative deuteration, and intra- and intermolecular radical addition can be achieved smoothly. Mechanistic studies indicate a single-electron photoredox catalytic system with inactivated solvent as the hydrogen atom donor.
- This article is part of the themed collection: 2019 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...