Atmospheric water triggers supramolecular gel formation of novel low molecular weight maslinic and oleanolic triterpenic derivatives†
Abstract
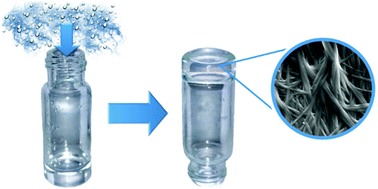
Pentacyclic triterpenes have a rigid lipophilic backbone decorated with polar functional groups such as hydroxyl, carbonyl and carboxyl, which make them attractive natural amphiphiles. The ability of this type of compound to self-assemble into complex nanostructures has recently gained interest due in part to the abundant existence of renewable feedstocks. Herein, we present two new maslinic and oleanolic triterpene pentacyclic derivatives that have the ability to self-assemble into long fibers giving rise to homogeneous gels. Quite remarkably, these molecules are able to form gels by slowly capturing atmospheric water from their DMSO and DMF solutions. To the best of our knowledge, this mechanism of gel formation has not been previously reported for other LMWGs and highlights the versatility of these compounds to form gels as a response to different external stimuli. The micro- and macroscopic properties of the resulting gels have been studied in detail by TEM, SEM, NMR, VCD, FTIR, XRD and rheology. We have demonstrated that gels prepared by capturing atmospheric water in DMSO are more homogeneous and stiffer than those obtained by direct addition of water to the organic solution.


 Please wait while we load your content...
Please wait while we load your content...