Modulating the crystallinity, mechanical properties, and degradability of poly(ε-caprolactone) derived polyesters by statistical and alternating copolymerization†
Abstract
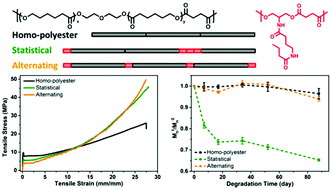
Poly(ε-caprolactone) (PCL) is a widely used biomaterial, but the long degradation time and hydrophobicity limit its applications in instances where short-term usage is needed. Synthesis of PCL analogues is an active area of research, but is constrained by the detailed and stringent synthetic procedures involved in the synthesis and polymerization of the cyclic lactones. Here we report a simple route to PCL analogues by copolymerizing PCL oligomer diol with a hydrogen bonding monomer diol and succinic acid in both statistical and alternating sequences. As a control, a homo-polyester from PCL oligomer diol and succinic acid was synthesized as well. The incorporation of the hydrogen bonding diol in different distributions results in correspondingly distinct polymer properties. The introduction of the hydrogen bonding monomer disrupts the microstructure of PCL which results in lower crystallinity, melting point and Young's modulus, but a more distinct strain hardening. The statistical distribution of the hydrogen bonding monomer along the polymer backbone accelerates the hydrolytic degradation rate.


 Please wait while we load your content...
Please wait while we load your content...
