(3S,4R)-3,4-Dihydroxy-N-alkyl-l-homoprolines: synthesis and computational mechanistic studies†
Abstract
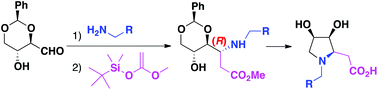
This is the first synthetic report of (3S,4R)-dihydroxy-N-alkyl-L-homoprolines described so far. 2,4-O-Benzylidene-D-erythrose was obtained from D-glucose with an improved yield, and then transformed into the title (3S,4R)-dihydroxy-N-alkyl-L-homoprolines, in a two-step strategy, with excellent overall yields. Hydrogenolysis of the benzyl group led to the NH congener. The synthesis of final products from 1,4-lactone intermediates was studied by computational means either under acidic or basic conditions. The theoretical mechanism studies fully explain the experimental results: (a) an equilibrium between L-homoprolines and their bicyclic counterparts is established in acids; (b) the equilibrium suffers a complete displacement towards the L-homoproline side in a basic medium.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...