A computational study of regioselectivity in aluminum hydride ring-opening of cis- and trans-4-t-butyl and 3-methylcyclohexene oxides†
Abstract
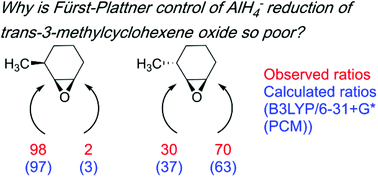
Nucleophilic ring opening of cyclohexene oxides is known to proceed preferentially through the trans-diaxial pathway (the Fürst–Plattner rule). This preference, however, is not absolute, and can be affected by substituents on the cyclohexene oxide ring, as illustrated by LiAlH4 ring-opening of the cis- and trans-isomers of 4-t-butyl- and 3-methylcyclohexene oxide (cis- and trans-1, cis- and trans-2). We performed B3LYP/6-31+G*(PCM) geometry optimizations to locate the chair-like and twist-boat-like transition structures for the hydride attacks on the pseudoaxial and pseudoequatorial conformers of these epoxides. Our calculations are consistent with the experimental observation of effective Fürst–Plattner control of AlH4−-opening of cis-1, trans-1, and cis-2, but low selectivity in ring-opening of trans-2. Our data at B3LYP/6-31+G*(PCM) suggests this reduction in selectivity is due to a diminished pseudoequatorial preference of the 3-methyl group in trans-2 relative to that in cis-2. The two calculated chair-like transition structures for hydride opening of trans-2 differ in activation energy free energy (ΔΔG‡) by only 0.4 kcal mol−1. Thus, these calculations account for the reduced regioselectivity of ring opening seen for trans-2 by AlH4− and other nucleophiles.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...
