Palladium(ii) ligated with a selenated (Se, CNHC, N−)-type pincer ligand: an efficient catalyst for Mizoroki–Heck and Suzuki–Miyaura coupling in water†
Abstract
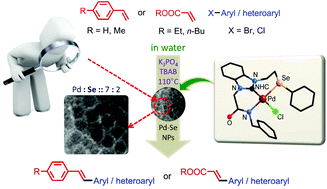
A new 1-[N-benzylacetamido]-3-[1-(2-phenylselenylethyl)]benzimidazolium chloride (L), the precursor of a novel (Se, CNHC, N−)-type pincer ligand (L) was synthesised in high yield through a sequence of consecutive reactions of 1H-benzimidazole with ethylene dichloride, sodium selenophenolate, and N-benzyl-2-chloroacetamide. The palladium-promoted reaction of L with PdCl2 resulted in a moisture- and air-insensitive complex [Pd(L–H2Cl)Cl] (1), which demonstrated outstanding catalytic potential for Mizoroki–Heck coupling of aromatic bromides and chlorides (with yields up to 94% and 70%, respectively) at very low catalyst loading (0.2 mol%) and under mild reaction conditions in water. The complex (1) was also investigated for Suzuki–Miyaura coupling and found to be selectively efficient (yields up to 94%) for Suzuki–Miyaura coupling of aromatic bromides at 0.01 mol% of 1 in water. All coupling reactions were carried out in the green and economical solvent, water, which is highly desirable for bulk synthesis of complex molecules in industry. During the catalytic process, complex 1 converted into PdSe nanoparticles (NPs, size range 5–6 nm) in situ. The morphology and composition of these NPs were analysed through high-resolution transmission electron microscopy and transmission electron microscopy-energy dispersive X-ray spectroscopy, respectively. The core-level, X-ray photoelectron spectroscopy analysis confirmed the presence of stable Pd0 and Pd2+ oxidation states in these PdSe NPs. Based on further experimental investigations, these nanoparticles were found to work as a stock of true catalytic species. The hot filtration test, as well as the two-phase test, confirmed the largely homogeneous nature of the catalytic process, which probably proceeds by leaching of solution-phase Pd species from these NPs.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC

 Please wait while we load your content...
Please wait while we load your content...