Bio-inspired enantioselective total syntheses of (−)-viminalins A, B, H, I, and N and structural reassignment of (−)-viminalin M†
Abstract
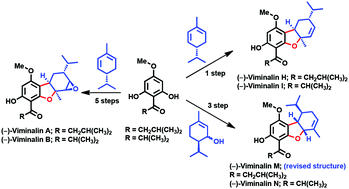
Bio-inspired enantioselective total syntheses of (−)-viminalins A, B, H, I, and N, isolated from the Myrtaceae family, were accomplished in a convergent fashion in 5, 5, 1, 1, and 3 steps, respectively. A highly regio- and diastereoselective oxidative [3 + 2] cycloaddition reaction of acylphloroglucinols with α-phellandrene, diastereoselective modified Friedel–Crafts reaction of acylphloroglucinols with piperetol, and stereoselective epoxidation of extremely hindered β-face were described as key reactions.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...