Robust synthesis of C-terminal cysteine-containing peptide acids through a peptide hydrazide-based strategy†
Abstract
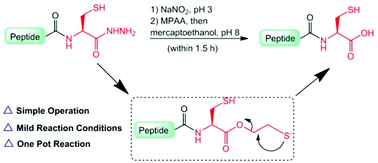
A new robust strategy was reported for the epimerization-free synthesis of C-terminal Cys-containing peptide acids through mercaptoethanol-mediated hydrolysis of peptide thioesters prepared in situ from peptide hydrazides. This simple-to-operate and highly efficient method avoids the use of derivatization reagents for resin modification, thus providing a practical avenue for the preparation of C-terminal Cys-containing peptide acids.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...