α-Thiocarbonyl synthesis via the FeII-catalyzed insertion reaction of α-diazocarbonyls into S–H bonds†
Abstract
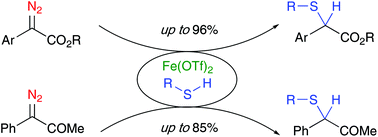
Fe(OTf)2 was used to catalyze the insertion reaction of α-diazocarbonyls into S–H bonds at 40 °C. A wide range of α-thioesters were obtained in yields up to 96% within 24–48 h from their corresponding α-diazoesters. A variety of thiols were used for the unprecedented insertion reaction with an α-diazoketone, leading to yields up to 85% of α-thioketones.
- This article is part of the themed collections: Synthetic methodology in OBC and Carbenes in Organic Synthesis


 Please wait while we load your content...
Please wait while we load your content...