Modular DNA-based hybrid catalysts as a toolbox for enantioselective hydration of α,β-unsaturated ketones†
Abstract
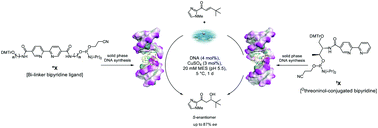
The direct addition of water to a carbon–carbon double bond remains a challenge, but such a reaction is essential for the development of efficient catalysts that enable direct access to chiral alcohols. We now report on the enantioselective hydration of α,β-unsaturated ketones, catalyzed by modular DNA-based hybrid catalysts, affording β-hydroxy ketones with up to 87% enantiomeric excess. Oligonucleotides containing an intrastrand bipyridine ligand were readily synthesized by a straightforward process using an automated solid-phase synthesis. A library of DNA-based hybrid catalysts could be systematically generated based on the composition of nucleobases, and the incorporation of a binding ligand and a nonbinding steric moiety. This study demonstrates the potential of modular DNA-based hybrid catalysts as a toolbox to accomplish the challenging enantioselective hydration reaction.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...