Pseudoenantiomeric glycoclusters: synthesis and testing of heterobivalency in carbohydrate–protein interactions†
Abstract
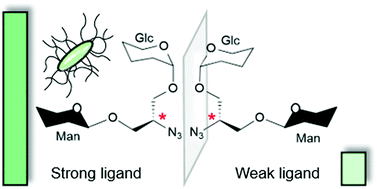
Multivalent carbohydrate–protein interactions are key events in cell recognition processes and have been extensively studied by means of synthetic glycomimetics. To date, frequently the valency, i.e. the multiplicity of the ligand attached to a polyvalent scaffold, has been considered in the design of multivalent structures but these studies have not led to a conclusive understanding of glycan recognition at the molecular level. In this work, we add a new aspect to carbohydrate–lectin recognition studies by designing the first heterobivalent diastereomeric glycoclusters in order to investigate the influence of both heteromultivalency and relative ligand orientation. Two enantiomeric scaffolds, derived from D- and L-serine, respectively, were glycosylated with two distinct carbohydrate ligands to obtain a library of pseudoenantiomeric glycoclusters. They all have an α-D-mannosyl residue in common as a specific ligand for lectins FimH and ConA, while they differ in the second carbohydrate portion, consisting of a β-D-glucosyl, a β-D-galactosyl or a β-D-glucosaminyl residue as unspecific ligands. The synthesised heteroclusters were tested in standard binding-inhibition assays investigating FimH-mediated bacterial adhesion and ConA binding to mannosylated surfaces. A striking difference was observed between the potencies of the two pseudoenantiomeric glucose-containing glycoclusters as inhibitors of FimH-mediated bacterial adhesion. For the other diastereomeric glycocluster pairs smaller inhibitory potency differences were detected in the bacterial adhesion assay. In contrast, the assays with ConA showed no significant variation for all tested cluster pairs. The results obtained with the diastereomeric glucose–mannose glycocluster pair were rationalised by molecular docking. Binding energies for the FimH carbohydrate recognition domain were calculated for both diastereomers and are in agreement with experimental data obtained in the bacterial adhesion assays.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...