Indium- and zinc-catalyzed enantioselective amide propargylation of aldehydes with stannylated allenyl amides†
Abstract
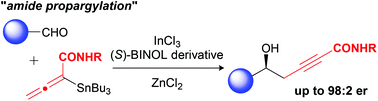
The catalytic enantioselective propargylation of aldehydes with newly prepared stannyl allenyl amides is described. The reaction has been accomplished by using catalytic amounts of indium chloride, zinc chloride, and a chiral BINOL derivative, affording amide-functionalized homopropargyl alcohols in excellent yields and enantioselectivities.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...