A novel approach to oxazole-containing diterpenoid synthesis from plant roots: salviamines E and F†
Abstract
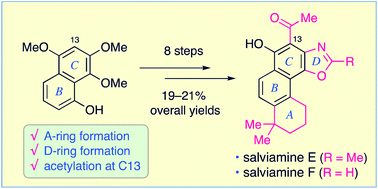
In this study, salviamines E and F, which are structurally unique abietane-type diterpene alkaloids containing an oxazole ring, were efficiently synthesized from a known molecule, 5,7,8-trimethoxy-1-naphthol. The synthetic sequence involves the following crucial steps: (i) the assembly of a carbon skeleton by coupling a six-carbon homoprenyl unit with a naphthalene moiety (Kumada–Tamao–Corriu coupling); (ii) the construction of a tricyclic phenanthrene ring by acid-induced cyclization of a naphthalene derivative with a homoprenyl side chain; (iii) the formation of an oxazole ring by nucleophilic ring closure of a 2-aminophenylene-1,4-diyl-diformate or -diacetate moiety and (iv) Friedel–Crafts acetylation at the C13 position of the tetracyclic intermediates to obtain the two target molecules, salviamines E and F. To the best of our knowledge, salviamine synthesis is reported here for the first time.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...