Synthesis of 1,3-disubstituted cyclohexenes from dienylethers via sequential hydrozirconation/deoxygenative cyclisation†
Abstract
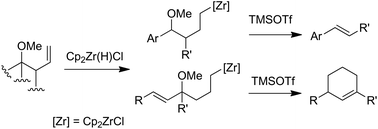
Access to 1,3-disubstituted cyclohexenes from zirconocenes containing a latent electrophilic allylic fragment is described. Requiring a specific conformation, 6-endo-trig cyclisation is based on the TMSOTf-mediated generation of a stabilized carbocation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...