Organocatalyzed asymmetric tandem conjugate addition–protonation of isocyanoacetates to 2-chloroacrylonitrile†
Abstract
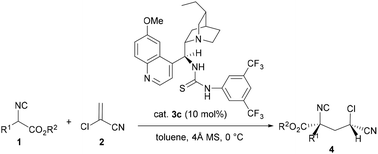
An efficient organocatalytic asymmetric tandem conjugate addition–protonation of α-substituted isocyanoacetates to 2-chloroacrylonitrile catalyzed by dihydroquinine-derived thiourea has been investigated, affording the corresponding adducts with two non-adjacent tertiary–quaternary stereocenters in excellent yields (up to 99%) along with good to excellent diastereo- and enantioselectivities (up to 20 : 1 dr, up to 95% ee) under mild conditions. The adduct can also be transformed into chiral γ-lactam by synthetic transformations.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...