Direct synthesis of 2,4,5-trisubstituted imidazoles from primary alcohols by diruthenium(ii) catalysts under aerobic conditions†
Abstract
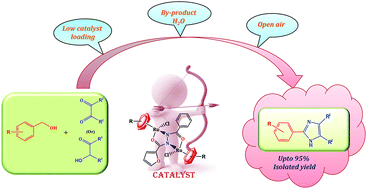
Herein we report a straightforward synthetic approach to 2,4,5-trisubstituted imidazoles from readily available primary alcohols using arene diruthenium(II) catalysts. Dinuclear arene ruthenium complexes have been synthesized and structurally characterized with the aid of analytical and spectral techniques. A library of 2,4,5-trisubstituted imidazoles was achieved with a yield up to 95% by loading 0.25 mol% of the catalyst. The present protocol is environmentally benign, which is performed under aerobic conditions and liberates water as the sole by-product.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...