Heterologous biosynthesis of a fungal macrocyclic polylactone requires only two iterative polyketide synthases†
Abstract
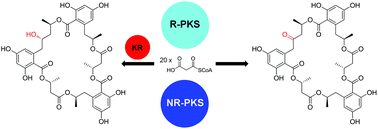
Menisporopsin A is a bioactive macrocyclic polylactone produced by the fungus Menisporopsis theobromae BCC 4162. A scheme for the biosynthesis of this compound has been proposed, in which reducing (R) and non-reducing (NR) polyketide synthases (PKSs) would catalyze the formation of each menisporopsin A subunit, while an additional non-ribosomal peptide synthetase (NRPS)-like enzyme would be required to perform multiple esterification and cyclolactonization reactions. Transcriptome analysis of M. theobromae identified an R-PKS gene, men1, and an NR-PKS gene, men2, which both exhibited highest expression levels during the menisporopsin A production phase. These were cloned into separate vectors for heterologous expression in Aspergillus oryzae NSAR1. Unexpectedly, coexpression of the two PKSs alone was sufficient to catalyze the formation of the macrocyclic polylactone, ascotrichalactone A, a structural derivative of menisporopsin A. The unanticipated esterification and cyclolactonization activities could reside in the unusual thioesterase domain of the NR-PKS, which is similar to that of the NRPS catalyzing elongation and cyclization of trilactone in enterobactin biosynthesis and that of modular PKSs catalyzing macrodiolide formation in elaiophylin and conglobatin biosyntheses.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...