Exploiting the vicinal disubstituent effect on the diastereoselective synthesis of γ and δ lactones†
Abstract
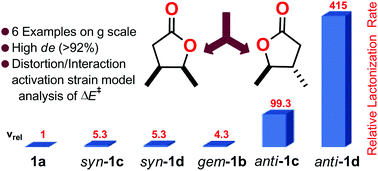
Trifluoroacetic acid catalysed lactonization of vicinal disubstituted γ-hydroxyesters was investigated in different solvents. The reaction kinetics, monitored by NMR spectroscopy, showed that: (i) the vic-disubstituent effect is stereoselective since the anti diastereoisomer ring closes substantially more rapidly than the syn isomer ring; (ii) the anti-vic effect is much stronger than the classical Thorpe–Ingold effect (known also as the gem-disubstituent effect), instead the syn diastereoisomers have rate constants comparable to that of the gem-disubstituted ester; (iii) the vic-effect can be enhanced by increasing the steric hindrance of one of the two substituents or carrying out the reaction in non-polar solvents. DFT computations of energy barriers (ΔG‡) were in good agreement with the experimental data. The distortion/interaction-activation strain model together with the Winstein–Holness kinetic scheme gave more insights into the origin of the vic-effect. An application of this effect consists of the diastereomeric resolution of disubstituted γ and δ lactones, among which are the naturally occurring Nicotiana t. lactone, the whisky and cognac oak lactones, and the Aerangis lactone. Both cis and trans diastereoisomers of these lactones were isolated in good yield and with high diastereomeric excess (de >92%). The selectivities of the diastereomeric resolution process, determined by NMR spectroscopy, are reported as well.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...