Substrate-directed chemo- and regioselective synthesis of polyfunctionalized trifluoromethylarenes via organocatalytic benzannulation†
Abstract
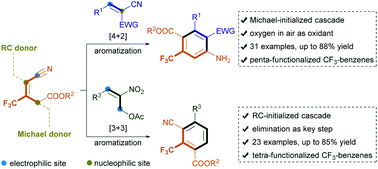
Highly chemo- and regioselective substrate-directed benzannulation of trisubstituted CF3-alkenes and 2-benzylidenemalononitriles or 2-nitroallylic acetates has been achieved via Michael-initiated [4 + 2] or Rauhut–Currier-initiated [3 + 3] annulation. These protocols enable the rapid assembly of structurally important tri-fluoromethylarene cores featuring five or four contiguous functional groups, affording a broad spectrum of products with diverse substituents in generally high yields. These products could be facilely transformed into structurally diverse molecules of synthetic and medicinal interest.


 Please wait while we load your content...
Please wait while we load your content...