Nanofibrillar hydrogels outperform Pt/C for hydrogen evolution reactions under high-current conditions†
Abstract
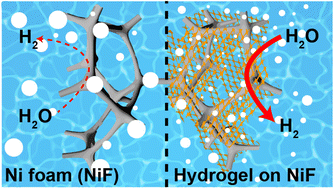
To achieve efficient hydrogen production, researchers have focused on the development of various electrocatalysts using noble and certain transition metal elements; however, concerns regarding their sustainability are growing. Here, we report a new methodology—hydrogel embedding—that significantly improves the performance of Ni foam (NiF) electrodes for alkaline hydrogen evolution reaction (HER) without the addition of any electrocatalysts. Hydrogel embedment significantly lowered the adhesion force of gas bubbles and imparted extremely bubble-repellent properties (i.e., superaerophobicity) to the underlying electrodes. Consequently, superaerophobic hydrogel-embedded NiF (HG-NiF) exhibited outstanding HER performance, even superior to that of NiF modified with commercial Pt/C catalysts under practically meaningful high-current conditions. For example, at −0.9 V vs. reversible hydrogen electrode, the HG-NiF and Pt/C NiF allowed current densities of −1.35 and −0.84 A cm−2, respectively. We demonstrate the practical applicability of superaerophobic hydrogels for efficient hydrogen production even without electrocatalysts made of environmentally less sustainable metallic elements.


 Please wait while we load your content...
Please wait while we load your content...