The role of gold oxidation state in the synthesis of Au-CsPbX3 heterostructure or lead-free Cs2AuIAuIIIX6 perovskite nanoparticles†
Abstract
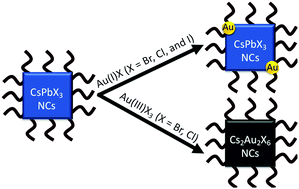
Here, we report that the oxidation state of gold plays a dominant role in determining the reaction products when gold halide salts are mixed with all-inorganic lead halide perovskite nanocrystals. When CsPbX3 nanocrystals react with Au(I) halide salts, Au nanoparticles are deposited on the surface of the perovskites through the reduction of Au1+ ions by the surfactant ligand shell, to produce Au-CsPbX3 heterostructures. These heterostructures preserve comparably high photoluminescence quantum yield (PLQY) and show identical XRD diffractograms as the parent CsPbX3 nanocrystals. In contrast, the reaction of CsPbX3 nanocrystals with Au(III) halide salts promotes complete cation exchange of Pb ions by Au ions in the nanocrystal perovskite lattice. The cation exchange products, Cs2AuIAuIIIBr6 or Cs2AuIAuIIICl6, show XRD patterns corresponding to a tetragonal mixed halide perovskite crystal structure and show no visible photoluminescence. This crucial dependence on the oxidation state of the Au ion informs synthetic strategies for producing and optimizing metal-perovskite heterostructures and lead-free perovskite nanoparticles.
- This article is part of the themed collection: Halide Perovskite Nanocrystals


 Please wait while we load your content...
Please wait while we load your content...
