Observation and implication of halide exchange beyond CsPbX3 perovskite nanocrystals†
Abstract
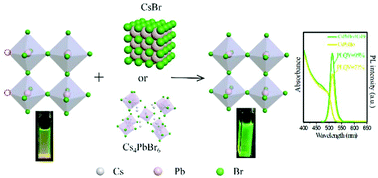
Anion exchange between pre-synthesized all-inorganic nanocrystals with a perovskite structure is a promising approach to tune their chemical composition and optical properties. Herein we have reported the first study of internanocrystal anion exchange reactionsin the cesium lead halide family, including CsPbX3, Cs4PbX6 and CsX, and we found that the anion exchange dynamics is highly dependent on their crystalline phase. In stark contrast to the fast rate in CsPbX3, cesium based non-perovskite NCs display much slower halide mobility. The reaction time is increased to several hours in Cs4PbX6 and days in CsX, respectively. Furthermore, we confirm that mixing these NCs with the same halide but different structures will induce halide diffusion from Cs4PbX6 NCs and CsX NCs to CsPbX3 NCs. This feature can be explored to utilize the Cs4PbX6 NCs and CsX NCs as a halide source to improve the photoluminescence efficiency and colloidal stability of CsPbX3 NCs.
- This article is part of the themed collection: Halide Perovskite Nanocrystals


 Please wait while we load your content...
Please wait while we load your content...