Syntheses, characterization, DNA/BSA binding, and in vitro cytostatic activity of fluorobenzenetelluronic triorganotin(iv) esters†
Abstract
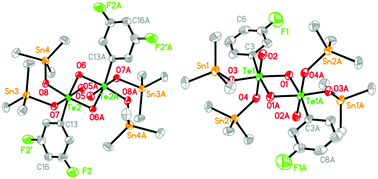
A new series of fluorobenzenetelluronic triorganotin(IV) esters, namely (R3Sn)4H2L (H6L1 = [3-FC6H4TeO(OH)3]2, R = Me: 1, R = Ph: 2; H6L2 = [3,4-F2C6H3TeO(OH)3]2, R = Me: 3, R = Ph: 4; H6L3 = [3,4,5-F3C6H2TeO(OH)3]2, R = Me: 5, R = Ph: 6), have been synthesized by the reaction of deprotonated fluorobenzenetelluronic acid ligands with the corresponding R3SnCl (R = Me, Ph). All the complexes have been characterized by means of elemental analysis, FT-IR, NMR (1H, 13C, 119Sn) spectroscopy and X-ray crystallography. Structural analyses of the complexes reveal that they are isostructural and display an almost planar four-membered Te2(μ2-O)2 core in the center. Each Te atom adopts a distorted octahedral geometry and each Sn atom adopts a distorted tetrahedral geometry. Complexes 1, 3, 5, and 6 form one-dimensional supramolecular structures, while 2 and 4 form two-dimensional supramolecular structures by intermolecular C–H⋯F or C–H⋯O interactions. Preliminary in vitro cytostatic studies show that these fluorobenzenetelluronic triorganotin esters exhibit effective cytostatic activity against a human cervical adenocarcinoma cell line (HeLa) and a human hepatocellular carcinoma cell line (HepG-2). For further discerning the apoptotic properties of cytostatic activity, the level of apoptosis was determined by flow cytometry (FACS assay). The DNA-binding properties of these complexes with calf thymus DNA (CT-DNA) and protein-binding properties with BSA were investigated by means of fluorescence titration. The results indicate that all the complexes could quench the intrinsic fluorescence of BSA in a static way.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...