Syntheses, structural diversity and photocatalytic properties of three coordination polymers assembled by different N-heterocyclic ligands†
Abstract
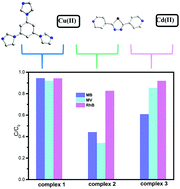
Three novel coordination polymers (CPs) based on transition metals, namely {[Cu(bpt)2(H2O)2]·2NO3}n (1), {[Cu(bpt)(SO4)(H2O)]·H2O}n (2) and [Cd(bpt)(SO4)]n (3) (tib = 1,3,5-tris(1-imidazolyl)benzene and bpt = 2,5-bis(4-pyridyl)-1,3,4-thiadiazole), have been designed and successfully synthesized under solvothermal conditions. All of these three polymers were characterized by single crystal X-ray diffraction, elemental analysis, IR spectra, powder X-ray diffraction (PXRD), and thermogravimetric analysis (TGA). Single-crystal X-ray diffraction analysis reveals that complex 1 possesses a uninodal 4-connected 3D (three-dimensional) spl topology framework with the point symbol (44·62). Complex 2 showing a 1D zigzag chain, which is connected by a sulfate anion to form a 2D network, is further assembled into a 3D supramolecular structure, whereas complex 3 displays a 3D 3,5-connected tfz-d topology with chiral inorganic 2-D layers. Moreover, the photocatalytic degradation of the three complexes has been investigated. Significantly, complex 1 is the best photocatalyst for the degradation of organic dyes (MB/MV/RhB).


 Please wait while we load your content...
Please wait while we load your content...