Switching of ionic conductivities in columnar liquid-crystalline anilinium salts: effects of alkyl chains, ammonium cations and counter anions on thermal properties and switching temperatures†
Abstract
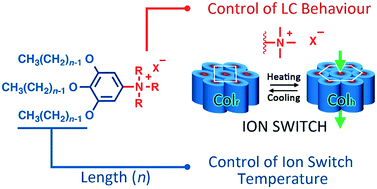
Switching one-dimensional ion conductivities in organic materials is a challenging task. Recently we found that a liquid-crystalline (LC) N,N,N-trimethyl-3,4,5-tridodecyloxy-anilinium salt showed a remarkable change in the conductivities of about four orders of magnitude, which was triggered by a thermotropic LC phase transition between rectangular (Colr) and hexagonal (Colh) columnar phases. In the present study, we have succeeded in tuning the switching temperature by changing the length of the alkyl chains attached to the anilinium ring of LC materials. The size of the N,N,N-trialkylanilinium cations, that is, trimethyl, dimethylethyl and diethylmethyl, and the counterion (BF4, PF6 or CF3SO3) play a crucial role in the formation of successive Colr and Colh LC phases, which affect the switching behaviour. We have found that the temperature of the Colr–Colh phase transition and the switching of ion conductivity are tuned in N,N,N-trimethylanilinium tetrafluoroborates by changing the length of the alkyl chains. These results give new insights into the design of ion-conductive self-assembled molecular materials.
- This article is part of the themed collection: Charge Transporting Nanostructured Polymers for Electrochemical Systems


 Please wait while we load your content...
Please wait while we load your content...
