The winding road of the uvaretin class of natural products: from total synthesis to bioactive agent discovery†
Abstract
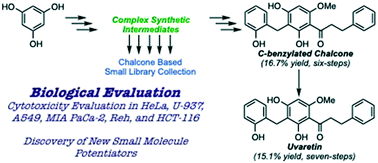
Herein, we disclose the development of a synthetic route to gain access to the uvaretin class of chalcone natural products. In this, the construction of a small library was achieved, and the collection was evaluated for cytotoxicity and other biological properties. Uvaretin (1) was accessed via a seven-step route in an overall yield of 15.1%. Within this route, the unsaturated enone variant of uvaretin (2), also a natural product, was accessed in a 16.7% yield over six steps. This route provides a nearly three-fold increase in yields of 1 and 2 in comparison to the previous synthetic route accessing them in 5.8% and 3.0% overall yields, respectively. Evaluation of 1 and 2 revealed IC50 values between 2.0 and 5.1 μM in the cancerous cell lines HeLa, U937, A549, and MIA PaCa-2. Screening of the whole chalcone library set led to the discovery of over 30 compounds, within six cancerous cell lines, possessing single digit μM IC50 activity as sole agents. Furthermore, multiple library members were found to possess promising potentiating properties with known chemotherapeutic agents.
- This article is part of the themed collection: Natural Products


 Please wait while we load your content...
Please wait while we load your content...