Electrochemical oxidative annulation of amines and aldehydes or ketones to synthesize polysubstituted pyrroles†
Abstract
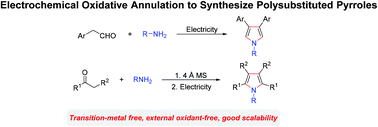
A general and practical protocol to synthesize polysubstituted pyrroles has been established by electrooxidative annulation of amines and aldehydes or ketones. In an undivided cell, arylacetaldehydes and primary amines can participate in this transformation smoothly to furnish β-substituted pyrroles under external oxidant-free conditions. Tetrasubstituted pyrroles could be obtained efficiently by using imines as substrates. In addition, this reaction can be extended to gram levels; the final products can also undergo late stage functionalization.


 Please wait while we load your content...
Please wait while we load your content...