Capacitive deionization using symmetric carbon electrode pairs†
Abstract
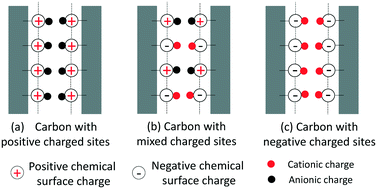
A commercially available carbon cloth electrode is repetitively oxidized using a wetting–drying procedure in order to enhance its negative chemical surface charge, consequently creating scenarios of symmetric electrode pairs with different potentials of zero charge (EPZC) versus potential at short circuit for ion desorption (Eo) in a potential distribution. In literature, this study of capacitive deionization (CDI) for the first time provides a new analytical approach that is capable of estimating the EPZC and potential distribution diagram. The approach leverages the modified Donnan model with chemical surface charge, in which the values of the chemical surface charge are quantified by investigating the effluent pH at steady state with and without carbon electrode pairs in a flow cell. Results from constant-voltage CDI tests show that, for a symmetric pair of carbon electrodes, the ion adsorption–desorption performance becomes efficient when a carbon electrode possesses equal positive and negative chemical surface charges or its net chemical surface charge is zero. By mapping EPZC and Eo in a potential distribution diagram, ineffective ion adsorption–desorption results from electronic charge consumed to repel co-ions at the intermediate charge storage stage. Additionally, Faradaic reactions at the electrodes are discussed based upon pH responses during CDI charging and discharging.


 Please wait while we load your content...
Please wait while we load your content...
