The heterogeneous reaction of dimethylamine/ammonia with sulfuric acid to promote the growth of atmospheric nanoparticles†
Abstract
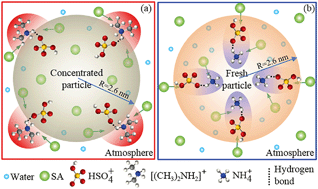
The growth of atmospheric nanoparticles plays key roles in a new particle formation (NPF) event. However, the interface heterogeneous reaction mechanism of dimethylamine (DMA)/ammonia with sulfuric acid (SA) to promote the growth of atmospheric nanoparticles remains unclear. In this work, an atmospheric nanoparticle model with 59 wt% SA was constructed, and the heterogeneous reactions of DMA/ammonia with SA in the air–nanoparticle interface as well as inside the bulk nanoparticle were comparably investigated with theoretical methods. The results revealed that the interface reaction mechanism of DMA to promote atmospheric nanoparticle growth is different from that of ammonia. DMA more readily approaches the air–nanoparticle interface than ammonia, which is more conducive to the occurrence of the heterogeneous reaction of DMA with SA in the interface than that of ammonia. In the DMA–SA–water system, the first solvation shell is composed of SA and water when DMA approaches the air–nanoparticle interface, and the DMA–SA cluster is subsequently formed in the air–aerosol interface. Accordingly, the efficiency of DMA in facilitating aerosol growth is independent from the concentration of gaseous SA. Moreover, DMA is more competitive in promoting the growth of concentrated aerosol than ammonia. In contrast, in the ammonia–SA–water system, the first solvation shell is re-composed of SA and water when ammonia is dissolved into the bulk nanoparticle, and the ammonia–SA cluster is formed. Furthermore, the growth of the nanoparticle depends on the concentration of gaseous SA. Considering the 2–3 orders of magnitude higher concentration of ammonia than DMA in the atmosphere, the growth of fresh aerosol is more easily promoted by ammonia than DMA. The conclusion that the growth of the nanoparticle was gradually promoted by the acidity of the first formed DMA–SA cluster during the NPF events was also obtained.


 Please wait while we load your content...
Please wait while we load your content...