Differences in photochemistry between seawater and freshwater for two natural organic matter samples†
Abstract
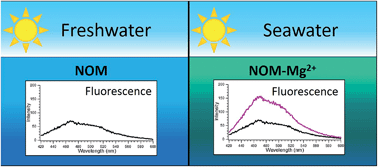
We report changes in the excitation and resolved fluorescence spectra, inferred triplet formation and singlet oxygen formation abilities of two different Natural Organic Matter samples (NOM) in seawater vs. freshwater or NaCl solution. In artificial seawater solution (but not in NaCl solution), the natural water-derived NOM samples Suwannee River Natural Organic Matter (SRNOM) and Nordic Reservoir Natural Organic Matter (NRNOM) display large enhancements in fluorescence intensity. Nearly identical spectra are seen when seawater is replaced by solutions of Mg2+ at its seawater concentration, consistent with magnesium binding to ligand sites of the natural organic matter giving rise to different photophysics. Fluorescence anisotropy measurements show a decrease in anisotropy of SRNOM and NRNOM in seawater, also consistent with Mg2+ binding. Different effects of Mg2+ are seen when the different NOM samples are illuminated: NRNOM exhibits increased formation of its triplet state and also quenching of its triplet by oxygen, compared to its photochemistry in the absence of Mg2+, while SRNOM exhibits a reduction in triplet formation in the presence of Mg2+. These observations imply that the photochemistry of NOM in seawater may be very different from what is expected based on freshwater or NaCl solution measurements.


 Please wait while we load your content...
Please wait while we load your content...