Trivalent copper stabilised by acetylacetone dithiocarbazate Schiff base ligands: structural, spectroscopic and electrochemical properties†
Abstract
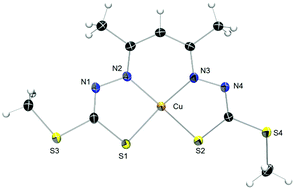
The copper coordination chemistry of N2S2 Schiff base ligands derived from acetylacetone and S-methyl or S-benzyl dithiocarbazate (H3acacsR, R = Me, Bn) reveals a rich variety of products depending on the reaction conditions. The free ligands spontaneously cyclise to their pyrazoline isomers but ring-open upon complexation with CuII. In the absence of oxygen, the ligands form CuIIN2S2 complexes ([CuII(HacacsR)]) that have been characterised electrochemically, spectroscopically and structurally. Intermediates in the complexation reaction are observed with time-resolved UV-Vis spectroscopy. Upon exposure to air, a number of different complexes are formed. Facile oxidation of [CuII(HacacsR)] to the trivalent analogue [CuIII(acacsR)] occurs in air. This compound is the precursor to two further oxidation reactions; one to the ketone [CuII(acacsRO)] where a carbonyl group has been installed at the apical C atom of the acetylacetone moiety and another to afford the novel dinuclear complex [(CuIII(acacsR))2]. The presence of excess base (Et3N) favours formation of the dimer.
- This article is part of the themed collection: Modern coordination chemistry


 Please wait while we load your content...
Please wait while we load your content...