Barium complexes with crown-ether-functionalised amidinate and iminoanilide ligands for the hydrophosphination of vinylarenes†
Abstract
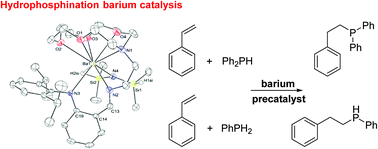
The detailed multistep syntheses of two nitrogen-based sterically congested iminoanilidine and amidine proligands bearing a tethered 15-member aza-ether-crown macrocycle, namely {I^Acrown}H and {Amcrown}H, are reported. These proligands react with [Ba{N(SiMe2H)2}2·(thf)n] to generate the heteroleptic barium complexes [{I^Acrown}BaN(SiMe2H)2] (5) and [{Amcrown}BaN(SiMe2H)2] (6) in high yields. These complexes exhibit high coordination numbers (resp. eight and seven) and are in addition stabilised by mild Ba⋯H–Si interactions. Unusually for oxophilic elements such as barium, the amidinate ligand in 6 is only η1-coordinated. Complexes 5 and 6 mediate the intermolecular hydrophosphination of styrene with primary (PhPH2) and secondary (HPPh2) phosphines. Their catalytic performance compares favourably with those of other barium precatalysts for these reactions. During the course of the hydrophosphination of styrene with HPPh2 catalysed by 5, the phosphide complex [{I^Acrown}BaPPh2] (7) could be intercepted and crystallographically characterised.
- This article is part of the themed collection: Nitrogen Ligands


 Please wait while we load your content...
Please wait while we load your content...