Alternative (κ1-N:η6-arene vs. κ2-N,N) coordination of a sterically demanding amidinate ligand: are size and electronic structure of the Ln ion decisive factors?†
Abstract
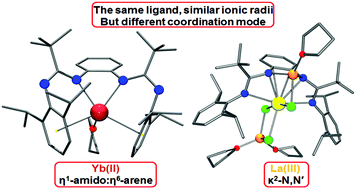
The amine elimination reaction of equimolar amounts of ansa-bis(amidine) C6H4-1,2-{NC(tBu)NH(2,6-iPr2C6H3)}2 (L1H) and [(Me3Si)2N]2Yb(THF)2 affords a bis(amidinate) YbII complex [C6H4-1,2-{NC(tBu)N(2,6-iPr2C6H3)}2]Yb(THF) (1) in 68% yield. Complex 1 features a rather rare η1-amido:η6-arene coordination of both amidinate fragments to the YbII ion, resulting in the formation of a bent bis(arene) structure. Oxidation of 1 by I2 regardless of the molar ratio of reagents (2 : 1 or 1 : 1) leads to the formation of the YbIII species [{(2,6-iPr2C6H3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC(tBu)NH}-C6H4-1,2-{NC(tBu)N(2,6-iPr2C6H3)}]YbI2(THF)2 (2) in which only one amidinate fragment is coordinated to the ytterbium ion in κ2-N,N′-chelating coordination mode, while the second NCN fragment is protonated in the course of the reaction and is not bound to the metal ion. The outcome of the salt metathesis reaction of LaCl3 with lithium amidinates [C6H4-1,2-{NC(tBu)N(2,6-R2C6H3)}2Li2] (R = Me, iPr) is proven to be strongly affected by the substituent 2,6-R2C6H3 on the amidinate nitrogens. When R = iPr, the salt metathesis reaction occurs smoothly and results in the formation of an ate-chloro-amidinate complex [C6H4-1,2-{NC(tBu)N(2,6-iPr2C6H3)}2]La(μ2-Cl)Li(THF)(μ2-Cl)2Li(THF)2 (3) in which the LaIII ion is coordinated by both amidinate fragments in a “classic” κ2-N,N′-chelating fashion. In the case of R = Me, the reaction requires prolonged heating for completion. Moreover, the salt metathesis reaction is accompanied by the fragmentation of the ligand and affords a trinuclear chloro-amidinate complex [C6H4-1,2-{NC(tBu)N(2,6-Me2C6H3)}2]La{[(tBu)C(N-2,6-Me2C6H3)2]La(THF)}2(μ2-Cl)4(μ3-Cl)2 (4) containing one dianionic ansa-bis(amidinate) and two monoanionic [(tBu)C(N-2,6-Me2C6H3)2] amidinate fragments. DFT calculations are conducted to determine the factor that governs this change in coordination mode and, in particular, the effect of the metal oxidation state.
NC(tBu)NH}-C6H4-1,2-{NC(tBu)N(2,6-iPr2C6H3)}]YbI2(THF)2 (2) in which only one amidinate fragment is coordinated to the ytterbium ion in κ2-N,N′-chelating coordination mode, while the second NCN fragment is protonated in the course of the reaction and is not bound to the metal ion. The outcome of the salt metathesis reaction of LaCl3 with lithium amidinates [C6H4-1,2-{NC(tBu)N(2,6-R2C6H3)}2Li2] (R = Me, iPr) is proven to be strongly affected by the substituent 2,6-R2C6H3 on the amidinate nitrogens. When R = iPr, the salt metathesis reaction occurs smoothly and results in the formation of an ate-chloro-amidinate complex [C6H4-1,2-{NC(tBu)N(2,6-iPr2C6H3)}2]La(μ2-Cl)Li(THF)(μ2-Cl)2Li(THF)2 (3) in which the LaIII ion is coordinated by both amidinate fragments in a “classic” κ2-N,N′-chelating fashion. In the case of R = Me, the reaction requires prolonged heating for completion. Moreover, the salt metathesis reaction is accompanied by the fragmentation of the ligand and affords a trinuclear chloro-amidinate complex [C6H4-1,2-{NC(tBu)N(2,6-Me2C6H3)}2]La{[(tBu)C(N-2,6-Me2C6H3)2]La(THF)}2(μ2-Cl)4(μ3-Cl)2 (4) containing one dianionic ansa-bis(amidinate) and two monoanionic [(tBu)C(N-2,6-Me2C6H3)2] amidinate fragments. DFT calculations are conducted to determine the factor that governs this change in coordination mode and, in particular, the effect of the metal oxidation state.
- This article is part of the themed collection: Nitrogen Ligands


 Please wait while we load your content...
Please wait while we load your content...