Cleavage of acyclic diaminocarbene ligands at an iridium(iii) center. Recognition of a new reactivity mode for carbene ligands†
Abstract
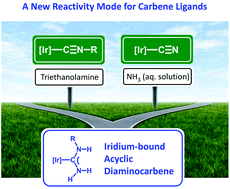
Reaction of [Ir(μ-Cl)(ppy)2]2 (1) with 4 equivs of CNC6H4X (X = F 2a, Cl 2b, Br 2c, I 2d) in the presence of 2 equivs of AgOTf in dichloromethane at 20–25 °C furnished the bisisocyanide complexes [Ir(ppy)2(CNC6H4X)2](OTf) ([3a–d](OTf); 72–87%). Reaction of [3a–d](OTf) with an excess of gaseous ammonia at room temperature gave the bisdiaminocarbene species [Ir(ppy)2{C(NH2)NHC6H4X}2](OTf) [5a–d](OTf) (73–83%); the two-step addition proceeds through an intermediate formation of appropriate monocarbene complexes [4a–d](OTf). Further reaction of [5a–d](OTf) with an excess of gaseous ammonia at 50 °C led to the cleavage of one diaminocarbene ligand to the cyanide ligand in [Ir(ppy)2(CN){C(NH2)NHC6H4X}] (6a–d) and this transformation is accompanied with the elimination of a substituted aniline. Treatment of [5a–d](OTf) with N(CH2CH2OH)3 at 50 °C resulted in the cleavage of the diaminocarbene ligand to the isocyanide and uncomplexed NH3; isocyanide remains bound to the iridium(III) center in [Ir(ppy)2{C(NH2)NHC6H4X}(CNC6H4X)](OTf) (4a–d). All isolated compounds were characterized by elemental analyses (C, H, N), molar conductivity measurements, TG/DTA, HRESI+/−-MS, FTIR, 1D (1H, 13C{1H}, 19F{1H}) and 2D (1H,1H-COSY, 1H,13C-HMQC/1H,13C-HSQC, 1H,13C-HMBC) NMR, and also by X-ray diffraction (for the bisisocyanide 3, the diaminocarbene/isocyanide 4, the bisdiaminocarbene 5, and the diaminocarbene/cyanide 6 type complexes).


 Please wait while we load your content...
Please wait while we load your content...