Chemistry and applications of cyanoximes and their metal complexes
Abstract
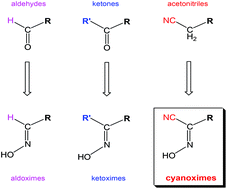
During the past three decades, considerable research effort has been dedicated to a new class of organic ligands – cyanoximes – which have the general formula NC–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOH)-R, where R is an electron-withdrawing group. The presence of the CN group makes cyanoximes ∼10 000 times more acidic and better ligands than other known conventional monoximes and dioximes. Also, in numerous cases, this group provides extra nitrogen donor atoms to support the formation of bridges between metal centres in the obtained coordination polymers. With 36 different R groups, the most abundant is the family of mono-cyanoximes, followed by 7 bis-cyanoximes which include aromatic and aliphatic spacers and, lately, tris-cyanoxime representing a ‘tripod’. The total number of obtained and characterized compounds is 44. These simple, low molecular weight molecules represent a series of new excellent ampolydentate ligands – ‘molecular Lego’, or building blocks – for coordination and organometallic chemistry. Uncomplexed ligands, their alkali metal salts, and metal complexes show a large spectrum of biological activity, ranging from growth regulation in plants and antimicrobial activity, to significant in vitro and in vivo cytotoxicity against human cancers. Currently, there are more than three hundred cyanoxime-based complexes, synthesized and studied using a variety of different spectroscopic methods and X-ray analysis. In this review, the preparation and stereochemistry of cyanoxime ligands, their structures and properties, and the most interesting coordination compounds with a broad spectrum of practical applications are summarized.
NOH)-R, where R is an electron-withdrawing group. The presence of the CN group makes cyanoximes ∼10 000 times more acidic and better ligands than other known conventional monoximes and dioximes. Also, in numerous cases, this group provides extra nitrogen donor atoms to support the formation of bridges between metal centres in the obtained coordination polymers. With 36 different R groups, the most abundant is the family of mono-cyanoximes, followed by 7 bis-cyanoximes which include aromatic and aliphatic spacers and, lately, tris-cyanoxime representing a ‘tripod’. The total number of obtained and characterized compounds is 44. These simple, low molecular weight molecules represent a series of new excellent ampolydentate ligands – ‘molecular Lego’, or building blocks – for coordination and organometallic chemistry. Uncomplexed ligands, their alkali metal salts, and metal complexes show a large spectrum of biological activity, ranging from growth regulation in plants and antimicrobial activity, to significant in vitro and in vivo cytotoxicity against human cancers. Currently, there are more than three hundred cyanoxime-based complexes, synthesized and studied using a variety of different spectroscopic methods and X-ray analysis. In this review, the preparation and stereochemistry of cyanoxime ligands, their structures and properties, and the most interesting coordination compounds with a broad spectrum of practical applications are summarized.
- This article is part of the themed collections: 2019 Frontier and Perspective articles and Nitrogen Ligands


 Please wait while we load your content...
Please wait while we load your content...