A new, simple, and efficient strategy for the preparation of active antifungal biodegradable materials via ring-opening polymerization of l-lactide with zinc aryloxides†
Abstract
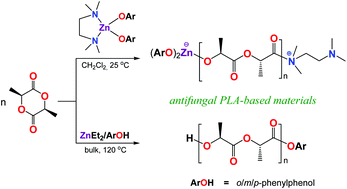
In this work, zinc aryloxides supported by monodentate hydroxybiphenyls [ArOH: ortho-phenylphenol (o-XenOH), meta-phenylphenol (m-XenOH), or para-phenylphenol (p-XenOH] and N,N,N′,N′-tetramethylethylenediamine (TMEDA) were used to develop active polymeric materials for antifungal agents for agricultural use. The direct reaction of ligand precursor ArOH with ZnEt2 (1 : 2) in a toluene/TMEDA mixture (1 : 10) afforded a series of three isostructural monomeric compounds, namely [Zn(o-XenO)2(TMEDA)] (1), [Zn(m-XenO)2(TMEDA)] (2), and [Zn(p-XenO)2(TMEDA)] (3). These were characterized by single-crystal X-ray diffraction, and spectroscopic and other analytical methods. The results show that 1–3 are effective initiators for the ring-opening polymerization (ROP) of L-lactide (L-LA) via bifunctional activation of the monomer with Lewis pairs to give polymers terminated with TMEDA and Zn(OAr)2 as the α- and ω-chain ends, respectively. Combinations of ZnEt2 with two molar equivalents of ArOH proligands were used to synthesize polylactides containing fungicide molecules covalently bonded via ester linkers. The ROP of L-LA initiated by these zinc-based systems could be used for the preparation of polyesters with promising antifungal activities.
- This article is part of the themed collection: Nitrogen Ligands


 Please wait while we load your content...
Please wait while we load your content...