Zn and Co redox active coordination polymers as efficient electrocatalysts†
Abstract
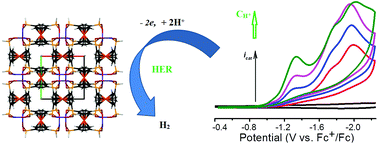
New redox active 1D helical coordination polymers M(fcdHp) (M(II) = Zn(1), Co(2)) have been obtained by utilizing the 1,1′-ferrocenylenbis(H-phosphinic) acid (H2fcdHp) ligand and Zn or Co nitrate salts. Complexes 1 and 2 are isomorphic, crystallizing in the chiral space groups P4122 and P4322, respectively. Their redox, electrocatalytic and other properties are described. These compounds incorporated into carbon paste electrodes and exhibited reversible redox reactions, arising from the ferrocenyl moiety. These coordination polymers are efficient as electrocatalysts for the reduction of protons to hydrogen. Using N,N-dimethylformamidium ([DMF(H)+]) as the acid in the acetonitrile solution, Co CP (2) displays a turnover frequency of 300 s−1, which is among the fastest rates reported for any CP electrocatalyst in CH3CN. This high rate of catalytic reaction comes at the cost of the 820–840 mV overpotential at the potential of catalysis. As the hydrogen evolution reaction (HER) catalysts, the CPs exhibited in 0.5 M H2SO4 the overpotential η10 of 340 or 450 mV, onset overpotential of 220 or 300 mV (vs. RHE), Tafel slope of 110 or 120 mV dec−1, correspondingly for 1 and 2, and considerable long-term stability for the HER.


 Please wait while we load your content...
Please wait while we load your content...