Synthesis and characterisation of homoleptic 2,9-diaryl-1,10-phenanthroline copper(i) complexes: influencing selectivity in photoredox-catalysed atom-transfer radical addition reactions†
Abstract
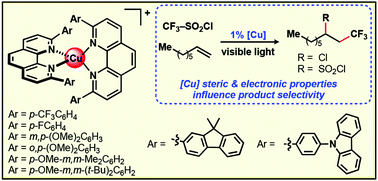
This report details the synthesis and characterisation of eight homoleptic bis(2,9-diaryl-1,10-phenanthroline)copper(I) complexes, seven of which are previously unreported {aryl = p-CF3C6H4, p-FC6H4, m,p-(OMe)2C6H3, o,p-(OMe)2C6H3, p-OMe-m,m-Me2C6H2, p-OMe-m,m-(t-Bu)2C6H2, 9,9-dimethyl-9H-fluoren-2-yl, 4-(9H-carbazol-9-yl)phenyl)}. Where possible the solid state, photophysical and electrochemical properties of these complexes were studied. In order to obtain insights into the influence of the intrinsic features of these copper(I) complexes on their reactivity in visible light-mediated photoredox catalysis, their capacity to promote a known atom-transfer radical addition process was evaluated. This specific transformation was identified as a suitable model system as it is reported to proceed via a mechanism consistent with the inner-sphere reactivity enabled by coordinatively unsaturated phenanthroline-based copper(I) species.
- This article is part of the themed collection: New Talent: Asia-Pacific


 Please wait while we load your content...
Please wait while we load your content...