Solid-state hydrogen rich boron–nitrogen compounds for energy storage
Abstract
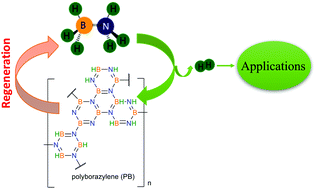
Boron compounds have a rich history in energy storage applications, ranging from high energy fuels for advanced aircraft to hydrogen storage materials for fuel cell applications. In this review we cover some of the aspects of energy storage materials comprised of electron-poor boron materials combined with electron-rich nitrogen elements with the goal of moderate temperature release of hydrogen. The parent compounds of ammonium borohydride, ammonia borane, and diammoniate of diborane provide approaches for storing high gravimetric and volumetric densities of hydrogen. Here we provide a review with a historical perspective and current developments in the area of solid state B and N containing compounds. This review highlights developments in synthesis of ammonia borane and its derivatives over the last 80 years. Thermodynamics and kinetics of hydrogen release in the solid state are discussed. By changing either substituents on the boron and nitrogen atoms or the physical environment by embedding in mesoporous scaffolds, the thermodynamics can be modified to reduce the exothermicity of hydrogen release and minimize formation of volatile impurities. Several mechanistic studies are reviewed identifying the key distinctions between homopolar and heteropolar H2 release. Strategies for economical and efficient regeneration of the hydrogen storage materials via chemical transformation are critically reviewed. The limited efficiency of these chemical regeneration has limited some of the potential applications.
- This article is part of the themed collection: Contemporary Research in Boron Chemistry


 Please wait while we load your content...
Please wait while we load your content...