Hydrogen- and oxygen-atom transfers in the thermal activation of benzene mediated by Cu2O2+ cations
Abstract
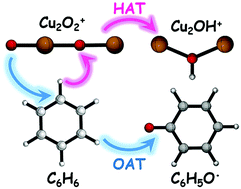
The mass-selected copper oxide cluster cations Cu2O2+ are successfully prepared by laser ablation and reacted with benzene in a linear ion trap reactor. The cluster reaction is characterized by reflectron mass spectrometry in conjunction with density functional theory calculations. Four types of reaction channels are observed: (1) Cu2OH+ + C6H5O˙, (2) Cu2C6H6+ + O2, (3) Cu(C6H6)2+ + Cu and (4) Cu2O2C6H6+, in which the first one is the major product. Observation of the products Cu2OH+ indicates that oxygen atom transfer (OAT) accompanying hydrogen atom transfer (HAT) occurs, and the oxygen-centered radical (O−˙) in Cu2O2+ is crucial to the cleavages of C–H and Cu–O bonds. It is interesting that HAT and OAT occur in a single reaction channel to form C6H5O˙, which has not been reported in the reactions of gas-phase ions with benzene. The calculated results are in agreement with the experimental observations, and no two-state reactivity scenario is present in the potential energy surface of channel 1. This work can provide useful insights into the nature of active sites over copper oxides and reaction mechanisms in the corresponding heterogeneous reactions.
- This article is part of the themed collections: 2019 PCCP HOT Articles and 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...