The Co2+/Ni2+ ion-mediated formation of a topochemically converted copper coordination polymer: structure-dependent electrocatalytic activity†
Abstract
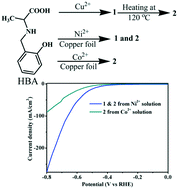
Surfactants or templating agents are known to influence the solid-state structural organization of metal–organic coordination polymers. Herein, we report the unusual influence of Co2+/Ni2+ ions on the formation of a copper coordination polymeric structure with the N-(-2-hydroxybenzyl)-alanine (HBA) ligand. The presence of Ni2+ ions in the growth solution led to two different structures: water-coordinated (1) and topochemically converted non-water coordinated (2) copper coordination polymer structures. On the contrary, the Co2+ ions led to only a non-water coordinated topochemically converted structure. X-ray absorption near-edge structure (XANES) and PXRD studies confirmed the absence of Co2+/Ni2+ ions in the network and the phase purity of the sample, respectively. The electrocatalytic hydrogen evolution reaction (HER) activity of copper coordination polymers in a basic medium (1.0 M KOH) revealed a relatively enhanced activity for the water-coordinated copper coordination polymer (jmax = 220 mA cm−2, η (10 mA cm−2) = 417 mV) as compared to that for the non-water coordinated structure (jmax = 84 mA cm−2, η (10 mA cm−2) = 535 mV). However, the mixed catalyst of 1 and 2 exhibited a relatively higher current density (jmax = 275 mA cm−2) as compared to the pure water-coordinated polymer (jmax = 220 mA cm−2). Importantly, the copper coordination polymer electrocatalyst did not require complementary conducting materials.
- This article is part of the themed collection: Editors collection: Metal Organic Frameworks as catalysts for water splitting and CO2 reduction


 Please wait while we load your content...
Please wait while we load your content...