Monitoring the hydrogen bond net configuration and the dimensionality of aniline and phenyloxamate by adding 1H-pyrazole and isoxazole as substituents for molecular self-recognition†
Abstract
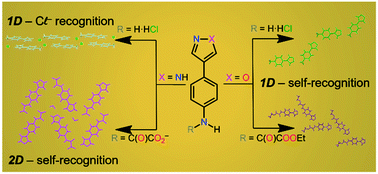
This work describes the synthesis and characterization of a new class of oxamic acid derivatives containing pyrazole and isoxazole as substituents to investigate their ability to form hydrogen bonds aiming at applying them in crystal engineering and molecular self-recognition. In this respect, we report a new synthesis of 2-(4-nitrophenyl)-1,3-propanedial (1) in high yield using SOCl2 as a chlorinating agent. The new oxamic esters 4-(1H-pyrazol-4-yl)phenylene-N-(ethyloxamate) (2d) and 4-(1,2-oxazol-4-yl)phenylene-N-(ethyloxamate) (3d) were prepared from 1. The synthetic route consists of the cyclisation of 1 either with hydrazine to afford 4-(-aminophenyl)-1H-pyrazole (2a) or with hydroxylamine to obtain the isoxazole-based molecule 4-(4-nitrophenyl)-1,2-oxazole (3a). The reduction of 2a and 3a was carried out in an acidic/tin solution to yield 4-(4-ammoniophenyl)-1H-pyrazol-2-ium trichlorostannate(II) chloride monohydrate (2b) and 4-(4-ammoniophenyl)-1,2-oxazole hexachlorostannate(IV) (3b). Basic extraction of 3b provided 4-(4-aminophenyl)-1,2-oxazole (3c). The reduction of 2a to 4-(4-aminophenyl)-1H-pyrazole (2c) was achieved by means of hydrazine associated with supported palladium on carbon. The condensation of 2c and 3c with ethyl chlorooxoacetate delivers oxamic esters 2d and 3d. In n-tetrabutylammonium hydroxide solution 2d is fully hydrolyzed, obtaining the n-tetrabutylammonium salt of 4-(1H-pyrazole-4-yl)phenylene-N-oxamate as a hemihydrate (2e). The low stability of isoxazole molecules in basic solutions was proved by crystallizing the n-tetrabutylammonium salt of 1-cyano-1-(4-nitrophenyl)-2-oxoethanide (3f) (obtained by cleavage of 3d with n-Bu4NOH) and preparing its conjugated acid 2-(4-nitrophenyl-3-oxopropanenitrile (3e). The structures of 2b, 3b, 3d and 2e were solved by single crystal X-ray diffraction techniques. The analysis of their crystal packing reveals hydrogen bond features compatible for all compounds as well as some differences depending on the pH of the crystallization solution and the presence or absence of the oxamate group due to the increase of hydrogen bond donors and acceptors.


 Please wait while we load your content...
Please wait while we load your content...